Department of Analytical Chemistry, Nutrition & Bromatology / Faculty of Chemistry/ University of Santiago de Compostela / 15782 Santiago de Compostela/ Spain
+34 981563100 / qncayebi@usc.es
Cespón Romero, Rosa Mª
Department of Analytical Chemistry, Nutrition & Bromatology / Faculty of Chemistry/ University of Santiago de Compostela / 15782 Santiago de Compostela/ Spain
+34 981563100 / rosacr@usc.es
ABSTRACT
ABSTRACT
A method based on an on-line ultrasound-assisted urine sample digestion procedure using a stopped-flow mode, followed by a flow injection preconcentration step using a minicolumn packed with a commercial chelating resin (Chelite P) is described for the determination of copper. Experimental designs were used as multivariate strategy for the evaluation at once of the effects of the variables involving digestion and preconcentration processes. Efficiency of the on-line total digestion-preconcentration process was studied in the µg/L range, obtaining quantitative recoveries with good precision (<5%). Finally, the proposed method was applied for determination of copper in urine samples from workers exposed to welding fumes and healthy unexposed volunteers (urine control).
Keywords
Keywords
Copper, urine, flow injection, flame atomic absorption spectrometry
INTRODUCTION
INTRODUCTION
The determination of metals as copper in urine is an important clinical screening procedure for diagnostics in numerous diseases and a suitable test for workers exposed to metal compounds [1]. Flame atomic absorption spectrometry (FAAS) still continues to be one of the most attractive approaches for trace metals analysis in most laboratories due to its relatively low operational costs, easy operation, and high sample throughput. However, the main two drawbacks for the direct determination of trace amounts of metals in urine samples by FAAS are low levels of metal ions and interferences of matrix components of urine samples. With the aim of solving these problems, continuous separation/preconcentration procedures involving a flow injection manifold with a minicolumn containing a chelating resin have been shown to be efficient and effective in enhancing the sensitivity and selectivity of FAAS [2-4]. In these procedures, an urine pre- treatment is mandatory to destroy the organic matter and decompose organic metallic compounds previous the preconcentration step.
In this work, we explore the benefits of ultrasounds for urine sample preparation by using an on-line system exploiting the stopped-flow mode. This novel system comprises a digestion unit connected to a FI preconcentration manifold, which is based on a minicolumn filled with a chelating resin (Chelite P, with aminomethylphosphoric acid groups). Thus, copper is on-line eluted and determined by FAAS. The present procedure has been applied to real urine samples of workers exposed to welding fumes for the determination of copper.
EXPERIMENTAL
Apparatus
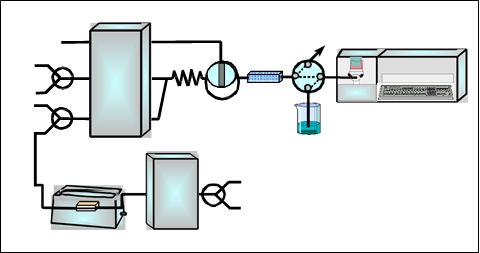
For flame atomic absorption determination of copper a Perkin Elmer Model 5000 air-acetylene flame atomic absorption spectrometer, attached to a Perkin Elmer Model 50 Servograph Recorder with a range of 5 mV was used. The flow injection (FI) system (Figure 1) used for the on-line urine sample pre-treatment and copper preconcentration was based on two Gilson Minipuls-3 peristaltic pumps fitted with Viton tubes, one injection valve and four switching valves, an ultrasonic bath and a glass minicolumn (100 mm x 15 mm i.d., bed volume 12 mL) used as digestion unit. The laboratory-made minicolumn for the on-line preconcentration step was prepared filling Viton tubes (100 x 1 mm i.d.) with 50 mg of chelating resin. All glassware and plasticware were decontaminated with 10% (V/V) nitric acid for at least 48 h and washed three with Milli-Q water before use.
 |
|
mL/min |
E
UW SV1
IV
MC MN
W SV3
0 .0 0 0
BS
UW SV2
DS P1
mL/min
Chelite P
UW
F A A S
UB SV4
DU
P2
B/UW S/SS
Figure 1. Continuous manifold for copper determination in urine samples. B: blank; BS: buffer solution; DS: digested sample; DU: digestion unit; E: eluent; FAAS: flame atomic absorption spectrometer; IV: injection valve; MC: mixing coil; MN: minicolumn containing the chelating resin (Chelite P); PP1 and PP2: peristaltic pumps; S/SS:sample or standard solution; SV1, SV2, SV3 and SV4: switching valves; UB: ultrasonic bath; UW: ultrapure water; W: waste
Chemicals and standard solutions
All reagents were of at least analytical grade. A copper stock standard solution of 1000 µg/mL (Merck, Germany) was used. Working standards solutions were prepared just before use by appropriate dilution of the stock standard solution. The nitric acid used to digest the urine sample and the hydrochloric acid used to elute copper from the chelating resin were from Merck. The chelating resin for copper preconcentration was Chelite P (Serva Electrophoresis, Germany; 20 mesh) with aminomethylphosphoric acid groups. The ammonium acetate (Merck) buffer solution (12 mol/L) was purified by passing it through a Chelite P minicolumn. Ultrapure water of 18.2 MΩcm resistivity, obtained from a Milli-Q water purification system (Millipore, Bedford, MA USA) was used for the preparation of the reagents and standards.
Samples and sample treatment
The urine of from workers exposed to welding fumes and from healthy unexposed volunteers was collected in polyethylene vessels. The urine specimens were filtered through a 0.45 µm Millipore membrane filter. Concentrated nitric acid was added to the samples, so that the samples are in a 4 mol/L nitric acid medium. The samples were stored in a refrigerator (~ 4 ºC) for no longer than 2 weeks.
Analytical Procedure
The FI system used for copper determination in urine samples is shown in Figure 1. In a first sep is carried out the urine sample digestion. Thus, the urine sample in 4 mol/L nitric acid medium is inserted into the flow system at 7 mL/min by means of a peristaltic pump (P2). Once all the sample volume is located in the digestion unit (DU), which is immersed in a ultrasonic bath at room temperature,the pump controlling the sample stream (B2) was stopped for a period of 3 min. After, the selecting valve (SV2) was switched to its other position, and pump B2 was again activated. In this way, the digested sample arrives at the part of the FI system where the preconcentration step takes place. For this, the digested sample stream converged with the buffer stream (12 mol/L ammonium acetate) in order to obtain the optimum pH value for copper retention in the chelating resin. Both channels are homogenised in the mixing coil and then, the resulting stream passed through the minicolumn containing the chelating resin (Chelite P). The sample matrix is sent to waste, while ultrapure water flowing through the detector. Finally, copper is eluted by injection of a volume of 110 µL of 3 mol/L hydrochloric acid into a water carried stream, which swept it to the FAAS detector where is continuously monitored.
In order to avoid carry-over, a washing step was including in the analysiscycle. Thus, the digestion unit was washed with ultrapure water during 30 s after each sample measurement.
RESULTS AND DISCUSSION
Optimisation of the preconcentration step
To determine the optimum values of the variables involving the preconcentration step, a study of these variables was performed for a synthetic urine matrix prepared by dissolving 17 g NaCl, 2.6 g NaH2PO4, 3.8 g KCl, 2.38 g Na2SO4, 0.72 g CaCl2 and 1.16 g MgCl2 in 2.7 L of ultrapure water [5]. This synthetic urine was spiked with copper (resulting concentration 50 µg/L Cu). A Plackett-Burman 2^7*3/32 factorial design with 12 runs plus one centerpoint was developed in order to determine the influence of the variables involving the preconcentration step. The factorial design was evaluated using the recovery as analytical response. The results demonstrated that the concentration of the ammonium acetate buffer channel is the only significant factor (with positive sign). The results of this screening design demonstrated that the variables need a final optimisation. In accordance with the recovery results: the preconcentration system is more efficient using the factor buffer channel flow-rate with the high level tested, while the mixing coil length and elution flow-rate do not affect the metal recoveries. Thus, these three factors were fixed at 2.0 mL/min, 100 cm and 4.0 mL/min, respectively. The other four factors (concentration of the buffer channel, sample flow-rate, and eluent volume and concentration) were optimised using an orthogonal central composite design, 2^4 + star with two centerpoints, resulting in26 randomised runs. These experiments were performed keeping all the other factors at values according to previous factorial screening design. The results obtained confirmed that the concentration of the buffer, sample flow-rate, and eluent concentration were statistically influential factors at the 95% confidence level in the ranges studied. Ammonium acetate buffer concentration and eluent concentration with positive sign and sample flow-rate with negative sign. While eluent volume is not a significant factor at the range studied. Given these findings, we decided to work with the following optimum operational conditions: 12 mol/L ammonium acetate buffer channel, sample flow-rate 2 mL/min, eluent volume 110µL, eluent concentration 3 mol/L.
Optimization of the on-line digestion procedure
The variables optimised in this step were the sonication time, digestion temperature and nitric acid concentration for the acid sample medium. The effects of these effects were simultaneously investigated using a 2^3 + star central composite orthogonal experimental factorial design with two centerpoints. The upper and lower values examined for each factor were sonication time: 1-3 min, digestion temperature: 20-70 ºC and nitric acid concentration for the acid sample medium: 1-4 mol/L). The axial distance value (α) between the centre of the design and the star points was 1.28719. This optimisation was developed by using an urine sample spiked with Cu (50 µg/L). Response (% recovery) was calculated against a classical off-line procedure involving urine sample digestion with nitric acid [6]. Theconclusions of this study were that sonication time and nitric acid concentration exceeded the level of statistical significance (95%) and the digestion temperature remained below the statistically significant level (95%) in the range under study. In light of the above results, it was decided to fix the upper values studied for significant factors. With regard to digestion temperature, room temperature (20 ºC) was selected as optimum for subsequent experiments because it has a very small estimated effect.
Analytical figures of merit
The characteristic performance data for the proposed method under the optimum conditions are presented in Table I. The preconcentration factor was calculated by the ratio of the slopes of the calibration curves obtained with and without preconcentration using FAAS, and the detection limit was calculated by the 3s criterion.
For method validation the recovery of the copper spiked into urine samples wasstudied. For this, increasing amounts of Cu were added to five urine samples randomly chosen, in such a way that the total Cu concentration remained within the linear range of the calibration curve. The average recovery was of 99.7±1.6 (range 98.1–101.3%), indicating the adequacy of the procedure for the determination of Cu in urine samples.
Table I
Analytical performance data of the on-line digestion/preconcentration method for copper determination in urine samples by FAAS

Preconcentration factor 42.6
Sample volume (mL) 5.0
Linear range (µg/L) 1.5-117.4
Regression equation (n=7); [Cu] in µg/L A = 2.6 x 10-3 [Cu]+ 3.0 x 10-4 Correlation coefficient r = 0.9998
Detection limit (3s) (µg/L) 0.5
Precision (RSD, n=11) (%) 0.5
Sampling frequency (samples/h) 9
Analysis of samples
The procedure developed in this work was applied to the determination of Cu in real urine samples. The copper concentrations found in the urine samples of the non-exposed persons (urine control) were in the range 5.7-28.4 µg/L, showing concordance with the concentration range reported in the literature (up to 50.0µg/L) [7]. The copper concentrations found in the urine of workers exposed to welding fumes were in the range 7.8-44.9 µg/L. These results are in agreement with those reported by Rainska et al., 2007, for urine of exposed workers [8].
CONCLUSIONS
An on-line procedure for determination of urinary copper is described, which combines sample digestion, preconcentration and copper determination by FAAS, which is available in most laboratories. The results obtained show that traces of copper can be accurately determined in a complex matrix sample like urine by a simple and inexpensive FI analytical methodology. The sampling frequency was of at least 9 samples per hour with minimum of sample preparation.
The results of sample analysis show that the urine from workers exposed to welding fumes has copper concentrations significantly elevated when are compared with the urine control. However, any sample presents a copper concentration higher than 50 µg/L.
REFERENCES
- 1. Schaller, K.H., Csanady, G., Filser, J., Juengert, B., & Drexler, H. (2007). Elimination kinetics of metals after an accidental exposure to welding fumes. International Archives of Occupational and Environmental Health (80) 7, 635 641
- 2. Anthemidis, A.N., Zachariadis, G.A., & Stratis, J.A. (2003). Gallium trace online preconcentration/separation and determination using a polyurethane foam mini column and flame atomic absorption spectrometry. Application in aluminum alloys, natural waters and urine. Talanta (60) 5, 929936
- 3. Almeida, A.A., Jun, X., & Lima, J.L.F.C. (2000). Flame AAS determination of copper in urine using a flow injection online preconcentration system based on a polyamine chelating ion exchange column. Atomic Spectroscopy (21) 5, 187 193
- 4. Aydemir, T., & Gucer, S. (1996). Determination of nickel in urine by flame atomic absorption spectrometry after activated carbon enrichment. Analytical Letters (29) 3, 351367
- 5. CespónRomero, R.M., & YebraBiurrun, M.C. (2008). Determination of trace metals in urine with an online ultrasoundassisted digestion system combined with a flowinjection preconcentration manifold coupled to flame atomic absorption spectrometry. Analytica Chimica Acta (609) 2, 184191
- 6. Maciel, C.J.C., Miranda, G.M., Palma de Oliveira, D., De Siquiera, M.E.P.B., Silveira, J.N., Leite, E.M.A., & Borba da Silva, J.B. (2003). Determination of cadmium in human urine by electrothermal atomic absorption spectrometry. Analytica Chimica Acta (491) 2, 231237
- 7. Caroli, S., Alimonti, A., Coni, E., Petrucci, F., Senofonte, O., & Violante, N. (1994). The assessment of reference values for elements in human biological tissues and fluids: A systematic review. Critical Reviews in Analytical Chemistry (24) 56, 363398
- 8. Rainska, E., Biziuk, M., Bogdan, J., Głombiowsk, P., Fodor, P., & Bielawsk, L.
(2007). Evaluation of occupational exposure in a slide bearings factory on the basis of urine and blood sample analyses. International Journal of Environmental Health Research (17) 2, 113-122
Papers relacionados