Introduction
In Brazil, Constitutional Amendment no. 49 of February 26, 2006 relaxed the regulations for the production and commercialization of short half-life radiopharmaceuticals, breaking the state monopoly and imposing on the domestic market a new paradigm, especially regarding the construction of radiopharmaceutical factories.
As the regulation of this industry is currently expanding, this study can effectively contribute to a set of specific national regulations that Will define minimum parameters to be followed in the construction process, with regard to occupational safety and health (OSH) during the production of radiopharmaceuticals, particularly in accordance with the rules of current health legislation (RDC 17/10; RDC 50/02).
One point to be considered is that the growth of nuclear medicine is directly related to the availability of radiopharmaceuticals in large quantities on the markets, and this in turn is directly linked to the construction of new radiopharmaceutical production factories, both private and public. The industrialization of these drugs (radiopharmaceuticals) must comply with sanitary requirements and radiological protection. With regard to OSH, the establishment of a single national standard is necessary, which would be in accordance with national and international health and radiological protection legislation, focused on OSH in the production lines of radiopharmaceuticals.
The objective of this study is therefore to propose a single standard in the form of directives that consider the aspects of Occupational Safety and Health for construction of radiopharmaceutical production factories in Brazil. It is hoped that this can contribute to the national economic growth, providing the industry with international competitiveness as well as promoting the safe and effective industrialization of these medicines according to the parameters of health and radiological protection, relevant and essential to the implementation of production activities.
Methodology
For ease of understanding and organization, the study methodology is divided into three phases.
Phase I consisted of accompanying the activities of a workgroup responsible for the supervision of construction of Pernambuco’s first radiopharmaceutical factory. The workgroup was responsible for analyzing the project plan and for accompanying its construction and licensing. The analysis was based on the set of architectural designs and special installations of the Radiopharmaceutical Production Unit implanted at the Northeast Regional Center for Nuclear Sciences.
The Standards used to analyze the Project from the point-of-view of occupational safety were: ISO series 14000, 18000, OHSAS series, especially 18001, regulatory Standards NR 15, 23, 24, and 32 of the MTE (Ministry of Labor and Employment), the health monitoring laws, especially RDC 63, 64, 50/02, and 17/10 of ANVISA (National Health Monitoring Agency), and the standards of CNEN (National Commission of Nuclear Sciences) for radiological safety and radioactive facilities: NE-6.02, NE-6.05, NE-6.06, NE-6.09, NE-5.01, NE-3.01, NE-2.01, and CNEN-10/96.
During the construction monitoring phase, the following regulatory standards were used: NR 05, 06, 07, 09, 18, 23, 24 and CONAMA Resolution 307.
The groups formed were divided according to their various specialties, constituting a multidisciplinary team composed of physicists, architects, engineers (civil, electrical, chemical, and occupational safety), and pharmacists.
Each group was responsible for the sectored design analysis using as a base the national and international standards pertaining to their specialties. Although many specialists had participated and conducted analyses, greater emphasis was given in this study to the construction phase (critical analysis of design/specifications) and to radiological safety, prioritizing occupational safety, which is principally described by its relevance to health and the lack, at the present time, of specific legal provisions that comprehensively address all of the characteristics of the pharmaceutical production factory construction process.
Phase II consisted of a technical visit to two domestic radiopharmaceutical production centers. In this manner, aspects of safety and radiation protection in the radiopharmaceutical production lines were observed, as well as constructive aspects and safety procedures implemented. Through observation of these aspects in both of the CNEN Institutes, the applicability of safety procedures, good radiopharmaceutical manufacturing practices, and the efficiency of the materials specified and used in areas of the production laboratories was observed.
The protocol was developed in the form of a synthetic and objective checklist, based on OSH and radiation protection standard, as well as on the aspects of construction considered. Below, the items observed at both radiopharmaceutical center installations contained in the verification protocol are detailed.
Unit data for radiopharmaceutical production – Basic information about radiopharmaceutical installations, laboratories, and cyclotron and hot-cell installations was collected.
OSH and radiological protection documentation – In this section, the existence of the minimum required documentation of OSH, radiological protection, and other written safety procedures at the visited installations was analyzed.
Physical facilities – The physical plant environment, accessibility criteria, disposition of the layout, positioning and Access of equipment, circulation and movement (flow) of material, electrical installations (substation, switchboard, and meter), water system facilities (differentiated sewer system, water tank), dressing barrier, air flow and air renewal used in the clean room environments, and the finishing materials used were observed.
Finally, phase III, which represents the scope of the work, was dedicated to establishing construction and safety directives, aiming to better control radiological hazards which workers classified as Individuals Occupationally Exposed (IOE) face, both in terms of the requirements of the construction Project as well as during the functioning of the radiopharmaceutical production facility.
Results
As described in the methodology sections, the phase of analysis and accompaniment of the construction project allowed for the detection of some existing nonconformities that hindered the project analysis and licensing of the site by government control and monitoring agencies.
During the accompaniment of the construction site in Pernambuco, some mishaps occurred with regard to the delay in sending the executive projects, the occurrence of alterations in the design, and the need to more rapidly consider the OSH conditions to contemplated at the worksite by the construction company. The accompaniment of the site occurred between September 2007 and November 2009, when the first radiopharmaceutical factory in northeastern Brazil was inaugurated.
The technical visits, along with the analysis of the relevant technical legislative framework, made it possible to evaluate compliance with OSH and radiation protection Standards in all phases of construction, including the selection and specification of materials used in the finishing phase.
Development of the radiopharmaceutical project analysis
The coordination of the multidisciplinary project analysis group, after receiving the intial design, analyzed it with respect to institutional standardization and good design requirements, approval and processing by legal authorities. The data from this analysis were recorded and any adjustment or alteration was passed on to the designers involved in the project.
The delivery mode for the service contract was of the “turn key” type, meaning that the completed structure should be delivered to the owners completely ready for use and fully licensed. The contract between the owner, in this case CNEN (the federal government), and the foreign company who owns the technology for development of radiopharmaceutical production factory projects used a local construction company as a contractor to perform the work.
In this phase, the compatibility project was begun, with participants including the group coordinator, the technical director of the department, the architect responsible, the accountant, the masonry and installation designer, the representative of the installation companies, and the engineer responsible for the construction.
Principal difficulties faced during project analysis
During the analysis of the radiopharmaceutical project, there was some difficulty in finding the relevant regulatory provisions required for project evaluation, so it became necessary to search for similar parameters in the context of factories designed for the production of injectable drugs, bringing together the environmental specifics in which radioactive material or substances are used.
During the project analysis, criteria of fire fighting and fire prevention, such as: corridors with adequate width, proper placing of fire-fighting and prevention equipment, fire extinguishers, hydrants, detection and alarm systems, existence and positioning of fire escape routes, lightning protection systems, and emergency illumination systems. Observations made with regard to sanitary conditions and workplace comfort, as well as the positioning and dimensioning of environments designed to meet workers’ needs for food and breaks throughout the day.
During project analysis, radiological protection criteria were also verified, such as: existence and positioning of collective protection equipment for the whole body and the environment, ionizing radiation detectors, emergency showers and eye-wash stations, wardrobe barriers, decontamination areas, physical intrusion security, and security of radioactive sources, through SFTV systems and observation points for property surveillance.
Accompaniment of the construction
The radiopharmaceutical architectural design was formed from a concrete bunker that houses the cyclotron, clean rooms, offices, and an engine room. The building was designed to meet all regulations from ABNT, ANVISA, for accessibility by disabled persons, as well for fire-fighting and prevention, aiming to provide users the perfect place to perform their tasks in a safe manner.
The technical specifications to be followed in the design will be mentioned below, in brief form, based on observation and experience at other radiopharmaceutical production centers as well as on the current recommended standards:
The design must contain a target cavern, cyclotron cavern, control room, power room, gas and compressed air room, machine shop, radioactive waste room, diesel generator room, electrical room – transformer and meter cabinet, an area for clean rooms, corridors, technical corridor, cleaning supply deposit, shipping area, both masculine and feminine bathrooms, bathroom for disabled persons, storage, quarantine room, general storage room, uninterruptible power supply and electrical cabinet room, data processing room, meeting room, secretary’s office, director’s office, other offices, radiation protection room, archives, break room, and center for ice water.
In the specific case of the Recife radiopharmaceutical factory, the clean room area is composed of changing rooms, reagent rooms, glass rooms, microbiological control rooms, quality control rooms, a FDG-18 production laboratory, a radiological protection room, packaging and shipping rooms, a decontamination room, an engine room with air conditioning machines, and a maintenance office
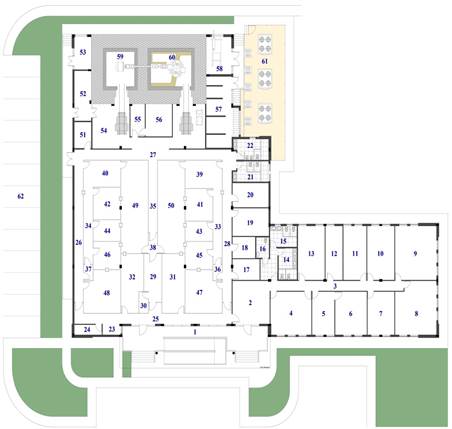
Figure 1. Floor plan of a radiopharmaceutical factory (CRCN-NE,2009)
Nonconformities detected during construction
Below are presented some of the principal nonconformities detected during construction, after due recommendations made in the design:
- Need for development of safety programs established by Brazilian legislation, namely: PPRA, PCMAT, PCMSO, PGRCC;
- Absence of firstaid kits;
- Deficiencies in construction site planning – need to forecast location of access ways, safety signage for pedestrian circulation and movement of material, delimitation of circulation areas and material storage, living areas, among others;
- Need for rodent extermination and control of pests and diseases (STDs, AIDS, dengue);
- Need for organization, uniforms, and hygiene during meal preparation for workers;
- Need for training in accident and disease prevention;
- Need for cleaning and organization of the worksite and living areas;
- Provisions for purchase of ergonomically certified furniture;
- Need to install an Internal Commission for Accident Prevention (CIPA) at the worksite;
- Need for improved working conditions during concrete pouring, especially for the bunker, accesses, catwalks, as well as overtime for nightshift workers;
- Need to control the effective use and replacement of individual protective equipment;
- Need for security and protection of machines and equipment;
- Need for safety and protection against electric shock, with the use of residualcurrent devices (RCDs).
Proposal for construction and OSH guidelines for the production of radiopharmaceuticals
According to the analysis performed, information was divided into two groups, one for the developer/builder and the other for the designer. A summary of the principal guildlines proposed follows.
Guidelines aimed at the developer/builder
These guidelines were formulated based on the needs of developers and builders, who will be involved in the planning and developing of projects for production of radiopharmaceuticals.
a) Carry out market research, involving the choice of suitable locations for the construction of a radiopharmaceutical production factory in accordance with standard regulation CNEN-NE-2.01;
b) Investigate the relationship between altitude, distance from the seas, topography, and historical rainfall of the region, in order to analyze the possibility of flooding and drainage of the land;
c) Choose the site (localization and situation with regard to neighboring structures) in accordance with the construction and urban planning codes of the municipality;
d) Perform topographic surveying that includes the location of boreholes for realizing soil surveys, verifying stability, nearness to mountains, and composition (clay, stone, sand, rock, etc.). For vertical stability, boreholes must be drilled in accordance with Brazilian technical Standards NBR 8036 and NBR 6502, which will aid in calculating the foundation of the building and its surroundings. This process should preferably be performed before the acquisition of the land, because there are cases where it is more feasible to acquire land with a price compatible with the region, than to invest in land that will require a high cost to prepare the foundation and site improvements;
e) Conduct a land survey, taking into consideration the special foundation structure, quality and uniformity of the land, and absence of organic materials (verify that the land was not a former landfill site);
f) Develop the draft project design, considering the cost of proper installation, the available labor, training costs, transportation, and other production factors, comparing them with the results of the market research already concluded.
g) Develop the project design by hiring a company/Professional specialized in this area (verify their experience with the CREA technical collection), based on regulations RDC 50/02, 307/02, 17/10, and 63/10, considering at a minimum:
- Designs for the construction, supplementation, renovation, or expansion of a building or set of buildings are developed, basically, in three steps: preliminary study, basic design, and executive design. The successive development of these steps will have, as a starting point, the program of physical and functional needs of the building, where the characteristics of the environments necessary for the development of the activities expected for the building;
- Preliminary study, basic design, executive design, in conformance with NBR 05679 and RDCs 17 and 63;
- Technical responsibility – The project author must sign all graphic design elements of the project, citing their registration numbers with the various government agencies and always providing the corresponding Technical Responsibility Annotation – ART, obtained in the jurisdiction where the project was designed. h) Obtain the initial licenses from the appropriate agencies: the water company, city hall, the electric company, IBAMA (environmental agency), the fire department, CNEN, and ANVISA; i) Hire a builder for the project, giving preference to companies with experience in this type of construction (verify their experience with CREA) and which prove the responsibility of their workers through OSH documentation and training, observing the contractual clauses referring to compliance with worker health safety requirements and standards, especially with regard to individual and collective protection, supply of appropriate PPEs, prevention of workrelated accidents and illnesses, etc.; j) Supervise and monitor the construction progress, through the use of personnel trained in civil engineering and occupational safety, making use of quality management system protocols and tools based on NR18, such as the “risk control and evaluation protocol for civil engineering”; k) Obtain the licensing for a radioactive installation from CNEN, in accordance with standard CNEN NE 6.02. l) Require that designs be modified at the conclusion of construction to match the structure “as built;” m) Upon completion of the construction, the company responsible should provide, based on Pernambuco State Law 278/2007, the manual for property owners and users. This law, to complement the civil defense and warning system referred to in Article 146 of the State Constitution, establishes basic rules for the obligatory performance of expert audits and periodic building maintenance, whether public (every three years) or private, as well as rules for preventive and/or corrective maintenance from damages to purchasers and users of the property, in terms of Article 5, XXXII and Article 24, VIII, both from the Federal Constitution. Article 6 of the same law provides that: builders deliver the manual for property owners and users at the same time as the delivery of the property itself. This manual contains, among other things, necessary and useful information in clear language about:
- All products and services used at the site, with the specifications, among others, of quantity, quality, expiration date, complete identification of producer and retailer including address, conditions of use and frequency of maintenance;
- Rules for property use, with special attention to the rules of safety and eventual hazards, among other, those relative to any modifications of the structure;
- The soil survey, with technical specifications, including the eventual treatment given as well as the foundation design;
- All executive engineering designs used in the construction of the building, together with their respective specifications, principally structural designs that objectively demonstrate how the building’s structure was constructed, as well as additional executive procedures related to other “as built” designs of the project;
- The ABNT standards regarding building safety and maintenance.
Guidelines aimed at the designer
These guidelines were formulated based on the needs of designers who are involved in the development of radiopharmaceutical production projects.
a) Develop the radiopharmaceutical factory design based on specific current Standards;
b) Provide criteria to insure OSH in the design of the project;
c) Monitor construction progress periodically, in order to guarantee the physical and financial status of the work and the quality of services performed;
d) Carry out design-construction interface, striving for clarity of information;
e) Present sufficient details in the design, with technical specifications of the materials to be used, descriptive histories of the various projects and specific designs:
- Design of special building systems;
- Blue print (layout);
- Design of structure and special foundations;
- Design of the layout of the installations;
- Design of Protection System Against Atmospheric Discharge (SPDA);
- Telephony/logic design;
- Design for firefighting and fire prevention, in accordance with local Fire Department Code and NR23;
- Physical protection design, in accordance with CNEN NE3.01;
- Façade/masonry design;
- Structural calculation design;
- Technological control of concrete;
- Airconditioning design;
- Design for special gases;
- Waterproofing design;
- Landscaping/environmental design;
- Illumination design (conforming to NBR 5413);
- Safety signage design, in accordance with NR18 and NR26;
- Construction site layout design, in accordance with NR18;
- PGRCC – Construction Waste Management Program;
- PCMAT – Work Environment Control Program for the construction industry, in accordance with NR18;
- Structural element design to ensure OSH during building maintenance work;
f) Provide a descriptive history of the project;
g) Provide updated design (as built) when delivering the completed work;
h) Adjust the design and reconcile the executive process with the design proposal.
Discussion of results
The construction process is, in general, composed of the planning phase, design phase, and execution phase. It was observed, however, that these phases unfold, containing internal phases, such as: consulting in the planning phase; analysis and approval of the design and licensing of the Project during the design phase; and accompaniment and monitoring of construction during the execution phase. There are a number of deficiencies across all of these phases that must be confronted when dealing with radiopharmaceutical production projects. Some difficulties detected are described below:
- The production of radiopharmaceuticals does not have Brazilian national reference standard in OSH nor civil construction. In the analysis phase of the radiopharmaceutical design, there was a certain level of difficulty in gathering the relevant regulatory provisions required for project analysis, making it necessary to search for similar parameters within the context of buildings designed for production of injectable drugs, bringing together the environmental specifics in which radioactive material or substances are used;
- The appropriate agencies do not have tools or personnel qualified RO analyze and approve construction projects of this nature;
- Developers, designers, and builders need technical information and minimum parameters for construction and OSH in order to develop this type of project;
These difficulties contribute to the lack of analysis of safety conditions throughout all phases of the constructive process, potentially creating a number of avoidable hazardous situations.
Therefore, it is recommended that OSH conditions be ascertained during the planning phase, analyzing the conditions in which the project will be executed, beginning with worksite planning and continuing through all of the construction phase as well as operation of the building. In this way, risk control and evaluation can be considered the planning phase, the constructive and OSH aspects can be observed, supporting effective and important tools that guarantee OSH conditions later during building operation and maintenance, such as:
With respect to the processing of the project by the legal review bodies for approval by local agencies, it is easy to note the shortage of qualified professionals with OSH training as well as specific knowledge of radiation protection and handling of nuclear materials and radioactive substances.
Regarding the project development at the actual construction site, there is also a distinct lack of experienced professionals with specific knowledge of OSH Who can implement and guarantee OSH actions, causing many instances of lack of compliance with legislative regulations, culminating in problems such as work-related accidents, embargoes, and closures.
The guidelines presented enable effective actions aimed at quality control of designs, during their preparation, as well as the reconciliation of designs during their coordination and critical analysis, favoring the systematization of information relative to the particularities of the production process and the building construction process.
It is very important that the enforcement agencies be in possession of scientifically-based tools that can guarantee the functioning of this type of structure in a manner safe for both workers and society.
Conclusion
The proposed guidelines consist of a summary of vital technical information that should be taken into consideration when developing a project whose main scope is the production of radiopharmaceutical medicine, in order to orient professionals such as developers, designers, builders, and others, aimed at guaranteeing the presence of OSH throughout all phases of a project including during the operational lifetime and maintenance of the building.
The recommendations of these guidelines arose from the needs identified during the early analysis phase of the first radiopharmaceutical production factory project in the State of Pernambuco. These were supplemented during visits made to two radiopharmaceutical production centers in Brazil, in order to observe their operation. These were corroborated during the accompaniment and monitoring of the construction work. They were established with the goal of creating a synthetic procedure that can be easily understood and used during construction by developers, designers, and builders.
The development of these guidelines is not intended to replace any requirement contained in the OSH or radiation protection legislation, nor in the standards applicable to construction. These recommendations are not binding and are not intended to replace or counteract existing laws and regulations.
Companies, whether builders or contractors, have an obligation to strictly comply with all worker safety standards and guarantee them at construction sites during all phases of a project, and should therefore provide the proper guidance to their employees, based on safety procedures for each specific activity and provide structural guidance to guarantee safe and healthy conditions for workers that perform operations or maintenance in the building.
The adoption of these guidelines is intended to provide a useful and accessible approach to fulfilling those responsibilities.
Bibliographic references
ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 8036: programa de sondagem de simples reconhecimento dos solos destinada à elaboração de projetos geotécnicos para a construção de edifícios. Rio de Janeiro, 1983.
ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 9050: acessibilidade a edificações, mobiliário, espaços e equipamentos urbanos. Rio de Janeiro, 2004.
ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 05679: elaboração de projetos de obras de engenharia e arquitetura. Rio de Janeiro, 1995.
ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 14037: manual de operação, uso e manutenção das edificações – conteúdo e recomendações para a elaboração e apresentação. Rio de Janeiro, 1998.
BARTH, I.; RIMPLER, A.; MIELCAREK, J. Beta radiation of medical personnel. In: of the Eleventh International Conference of the IRPA, 22-28 May 2004, Madrid. Proceedings…Spain (CD-ROM), 2004.
BRASIL. Casa Civil. Lei nº 8987, publicada no DOU de 13 fev 1995.
BRASIL. Decreto-lei nº 3365, (Lei Geral de Desapropriações), publicado no DOU de 21 Jun 1941.
BRASIL. Ministério da Previdencia Social. Saúde e segurança ocupacional. Available at: <http://www.previdenciasocial.gov.br/conteudoDinamico.php?id=39>. Accessed on: 10 Sep. 2010.
BRASIL. Ministério do Trabalho e Emprego. Normas Regulamentadoras. Available at::<http://www.mte.gov.br>. Accessed on: 10 Fev. 2009.
BRASIL. Lei n.8.213, de 24 de julho de 1991. Dispõe sobre os Planos de Benefícios da Previdência Social e dá outras providências. DOU de 25/07/1991. Available at: <http://www81.dataprev.gov.br/sislex/paginas/42/1991/8213_7.htm.> Accessed on: 15 Jan. 2010.
BRITISH STANDARDS INTERNATIONAL. OHSAS 18001: occupational health and safety management systems (Specifications). London, 1999.vv
COMISSÃO NACIONAL DE ENERGIA NUCLEAR. Posição Regulatória 3.01/004. Available at: <http://www.cnen.gov.br/seguranca/normas/pr301_004.pdf>. Accessed on: 29 mar. 2010.
COMISSÃO NACIONAL DE ENERGIA NUCLEAR. Diretrizes básicas de radioproteção – CNEN-NE-3.01. Rio de Janeiro: 1988.
COMISSÃO NACIONAL DE ENERGIA NUCLEAR. Serviços de radioproteção – CNEN-NE-3.02. Rio de Janeiro: 1988.
COMISSÃO NACIONAL DE ENERGIA NUCLEAR. Diretrizes básicas de proteção radiológica – CNEN-NN-3.01. Rio de Janeiro: 2005.
CONSELHO NACIONAL DO MEIO AMBIENTE. Resolução n. 307 de 5 de julho de 2002. Estabelece diretrizes, critérios e procedimentos para a gestão dos resíduos da construção. Diário Oficial da República Federativa do Brasil.
DE CICCO, F. Manual sobre gestão da segurança e saúde no trabalho: A primeira “Norma” de âmbito mundial para certificação de sistemas de gestão da SST. São Paulo: Risk Tecnologia, 1999. v. 3: OHSAS 18001.
DIAS, L. M. A. Training of Construction Safety and Health Coordinators: an experience in the European Union. In: A Global perspective: construction safety education and training. Flórida: International e-Journal of construction, 2003.
HINZE, J. Construction safety. Englewood cliffs: Prentice-Hall, 1997, 331p.
INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION. Recommendations of the international commision on radiological protection. (Adopted september 9, 1958). Pergamon Press, London, (1959). (ICRP Publication 1).
INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION. Report of Committee II on permissible dose from internal radiation. (1959). Pergamon Press, London, In press, (ICRP Publication 2).
INTERNATIONAL ATOMIC ENERGY AGENCY. Basic safety standards for radiation protection. IAEA, Vienna, 1962 (Safety Series No. 9).
INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION. Recommendations of the international commission on radiological protection. (Adopted september 17, 1965). Pergamon Press, Oxford, (1966). (ICRP Publication 9).
INTERNATIONAL ATOMIC ENERGY AGENCY. Basic safety standards for radiation protection. IAEA, Vienna, 1967 (Safety Series No. 9).
INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION. Implication of commission Recommendations that doses be kept as low as readily achievable. ICRP, Viena, 1973 (ICRP Publication 22).
INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION. Recommendations of the international commission on radiological protection. ICRP, Viena, 1977 (ICRP Publication 26).
INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION. Limits for intakes of radionuclides by workers. (1978). Pergamon Press, London, (ICRP Publication 30).
INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION. General principles of monitoring for radiation protection of workers. ICRP, Viena, 1982 (ICRP Publication 35).
INTERNATIONAL ATOMIC ENERGY AGENCY. Basic safety standards for radiation protection. IAEA, Vienna, 1982 (Safety Series No. 9).
INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION. General principles of monitoring for radiation protection of workers. ICRP, Viena, 1968 (ICRP Publication 12).
INTERNATIONAL ATOMIC ENERGY AGENCY. The basic requirements for personnel monitoring. IAEA, Vienna, 1965 (Safety Series No. 14).
INTERNATIONAL ATOMIC ENERGY AGENCY. Basic requirements for personnel monitoring. IAEA, Vienna, 1980 (Safety Series No. 14).
INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION. Cost benefit analysis in the optimization of radiation protection. ICRP, Viena, 1982 (ICRP Publication 37).
INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION. Optimization and decision-making in radiological protection. ICRP, Viena, 1988 (ICRP Publication 55).
INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION. Recommendations of the international commission on radiological protection. ICRP, Viena, 1991 (ICRP Publication 60).
INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION. Principles for the radiation protection of workers. ICRP, Viena, 1997 (ICRP Publication 75).
INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION. The 2007 Recommendations of the International Commission on Radiological Protection. Oxford: Elsevier Ltd. (ICRP Publication 103).
INTERNATIONAL ATOMIC ENERGY AGENCY. Basic principles for occupational radiation monitoring. IAEA, Vienna, 1987 (Safety Series No. 84).
INTERNATIONAL ATOMIC ENERGY AGENCY. International basic safety standards for protection against ionizing radiation and for the safety of radiation sources. IAEA, Vienna, 1996 (Safety Series No. 115).
SANTOS-OLIVEIRA, R.; BENEVIDES, C. A.; KOHLMAN RABBANI, E. R.; RICARTE FREITAS, I. M. A. T. Radiopharmaceuticals Industry based on the Brazilian regulamentation. FABAD J. Pharm. Sci., 33, 189-194, 2008.
Papers relacionados